Who We Are
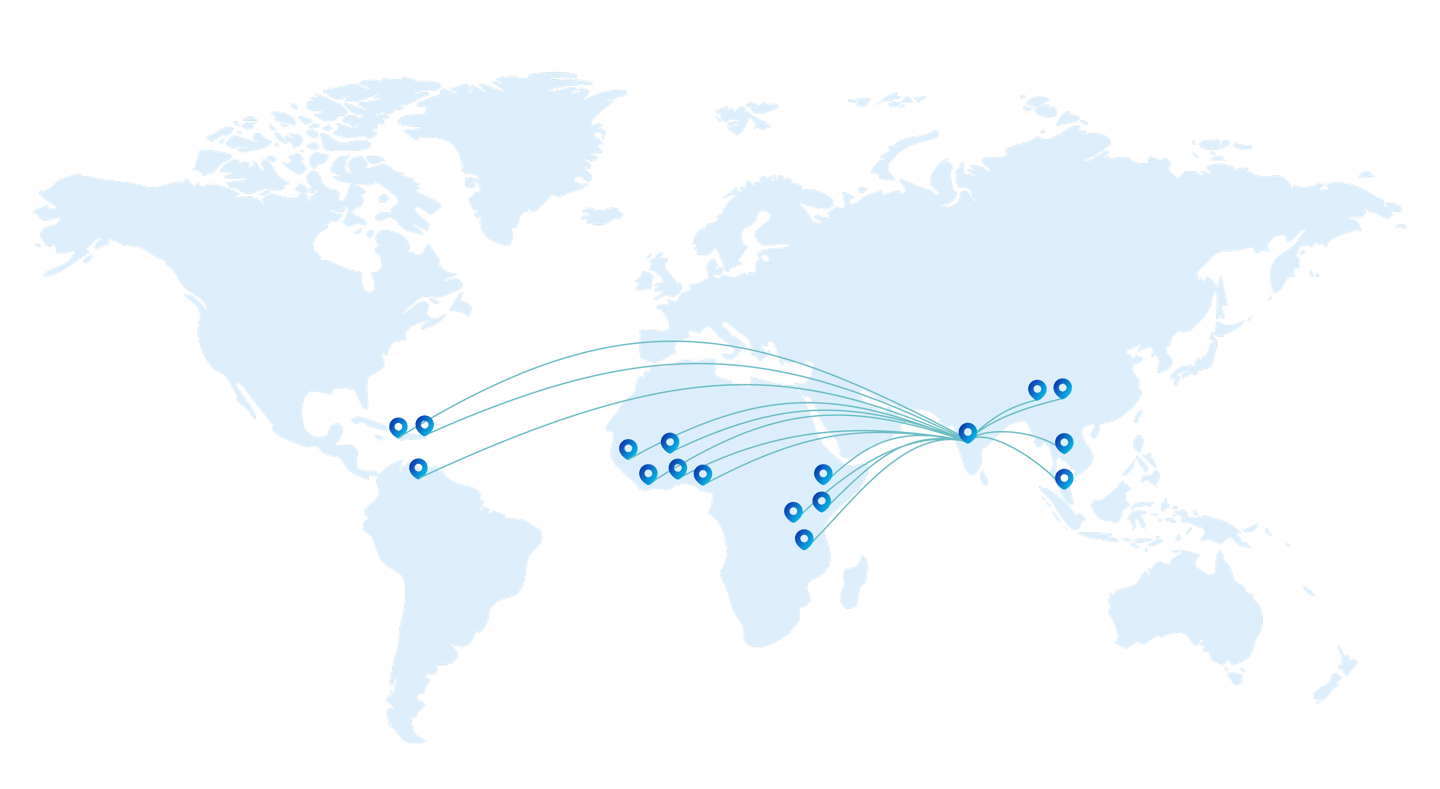
Founded in 2008 and based in Goa, India, D.K. Enterprises is a trusted name in the pharmaceutical export industry. With a presence in over 16 countries, we are driven by a mission to support business development in global pharmaceutical markets. The company is led by seasoned professionals with more than 25 years of experience in pharmaceutical business development and international marketing.
16
25+
Years of experience
Countries
45+
Products
We began our operations as export consultants and exporters, helping Indian pharmaceutical manufacturers tap into global opportunities. Over the years, we have grown to develop and market our own branded pharmaceutical products, manufactured at WHO-GMP certified facilities in India. In the past three years, we have introduced and successfully launched over eight pharmaceutical brands in African markets through strategic partnerships.
Our Journey
What we do
At D.K. Enterprises, we serve both Indian suppliers and international buyers.
For Indian clients, we identify export opportunities, connect them with the right global buyers, assist with product registration, and support the finalization of export orders.
For international clients, we act as sourcing agents - procuring quality-assured pharmaceutical products from approved WHO-GMP certified manufacturers in India, tailored to specific market requirements.
Manufacturing Excellence
All our manufacturing facilities are equipped with modern machinery and operate under rigorous quality control systems. We are capable of delivering products in a wide variety of dosage forms and batch sizes, ensuring flexibility and scalability to meet diverse market needs. Every facility is certified by WHO-GMP, reflecting our commitment to global quality standards.
Research & Development
Our R&D division plays a vital role in maintaining product quality and innovation. It focuses on:
Developing new drug formulations
Enhancing manufacturing processes
Improving cost-efficiency, process consistency, and quality control
This continuous innovation helps us stay ahead in an evolving industry.
Production Capacity
We maintain a robust production capacity to meet large-scale global demands:
Tablets: 60 million/month
Capsules: 30 million/month
Liquids: 1.5 million bottles/month
Injections: 1.5 million ampoules & 0.5 million vials/month
Oral Suspensions: 2.5 million bottles/month
Ointments: 1.5 million tubes/month
Sterile Ophthalmic: 1.5 million bottles/month